

In dairy analysis, the applications of infrared spectroscopy encompass two main areas: (1) quantification of concentrations and quality parameters and (2) testing of purity, identity, and adulteration. Its adoption for food and dairy analysis is increasing at a tremendous pace driving the demand for better instrumentation, miniaturization, and portability to enable on-site analysis for immediate results. It offers speed, convenience, reliability, and accuracy to the analyst, and reduces cost, time, and labor in the industry. Infrared spectroscopy monitors the interaction of functional groups in chemical molecules with infrared light resulting predictable vibrations that provides a “fingerprint” characteristic of chemical or biochemical substances present in the sample. Rodriguez-Saona, in Reference Module in Food Science, 2016 Abstract With numerous developments in FT-IR spectroscopy and several applications still unexplored, the future of IR spectroscopy in dairy analysis and control is promising.Ī. Another recent development is the miniaturization of FT-IR instrumentation (e.g., TruDefender™ FT handheld FT-IR by Ahura Scientific, Inc., shown in Fig. 6), which would enable on-site analysis while the products are being produced.
A relatively new IR imaging technique, which constructs a complete image of the sample with each point in the image containing a complete spectrum, can offer advanced features for dairy research. Coupling IR spectroscopy with analytical techniques such as gas chromatography, liquid chromatography, and thermogravimetric analysis has extended its analytical capabilities. Raman spectroscopy is not as susceptible to water as IR spectroscopy and may be suitable for some applications. The most significant advancement in IRS has resulted, however, from the introduction of Fourier transform (FT) instruments that has made IRS even more rapid, reproducible, and sensitive.Īlternative spectroscopic techniques or coupling IR spectroscopy with other methods may also be required to achieve the desired outcome. The earliest applications of IRS were reported in the 1950s, but it was not until the 1960s that IRS was used for the analysis of food samples. The first IRS instrumentations became available in the late 1940s. IRS has also widened the horizons for the detection of adulteration and the authentication of food products. This makes IRS a potent tool for the quality control/assurance in a variety of different (industrial) settings and suitable for applications under steady process conditions, that is, on-line. IRS provides qualitative and quantitative information in a fast, cost-effective and nondestructive way, does not require the use of polluting chemicals, and can be carried out even by minimally trained personnel. Infrared spectroscopy (IRS) is one of the most important analytic techniques available to food scientists and industry nowadays. van Ruth, in Encyclopedia of Food and Health, 2016 Introduction Journal of Physical and Chemical Reference Data. "Vibrational and Electronic Energy Levels of Polyatomic Transient Molecules. ^ NSRDS-NBS: National Standard Reference Data Series, National Bureau of Standards (PDF).Infrared and Raman Spectra of Inorganic and Coordination Compounds, Applications in Coordination, Organometallic, and Bioinorganic Chemistry. Infrared and Raman Spectroscopy Principles and Spectral Interpretation. Infrared and Raman Characteristic Group Frequencies: Tables and Charts. Two bands (distinct from ketones, which do not possess a C─O bond) Influenced by conjugation and ring size (as with ketones) Influenced by conjugation (as with ketones) Tables of vibrational transitions of stable and transient molecules are also available. IR spectroscopy is useful when it comes to analysis of inorganic compounds (such as metal complexes or fluoromanganates) as well.

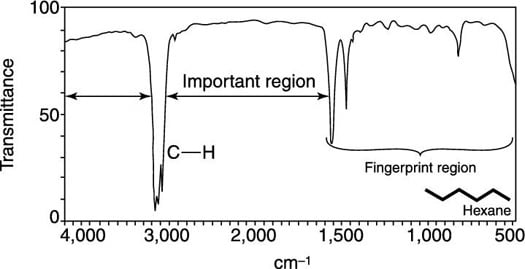
The absorptions in this range do not apply only to bonds in organic molecules. In physical and analytical chemistry, infrared spectroscopy (IR spectroscopy) is a technique used to identify chemical compounds based on the way infrared radiation is absorbed by the compound. Further information: Infrared spectroscopyĪn infrared spectroscopy correlation table (or table of infrared absorption frequencies) is a list of absorption peaks and frequencies, typically reported in wavenumber, for common types of molecular bonds and functional groups.


 0 kommentar(er)
0 kommentar(er)
